|
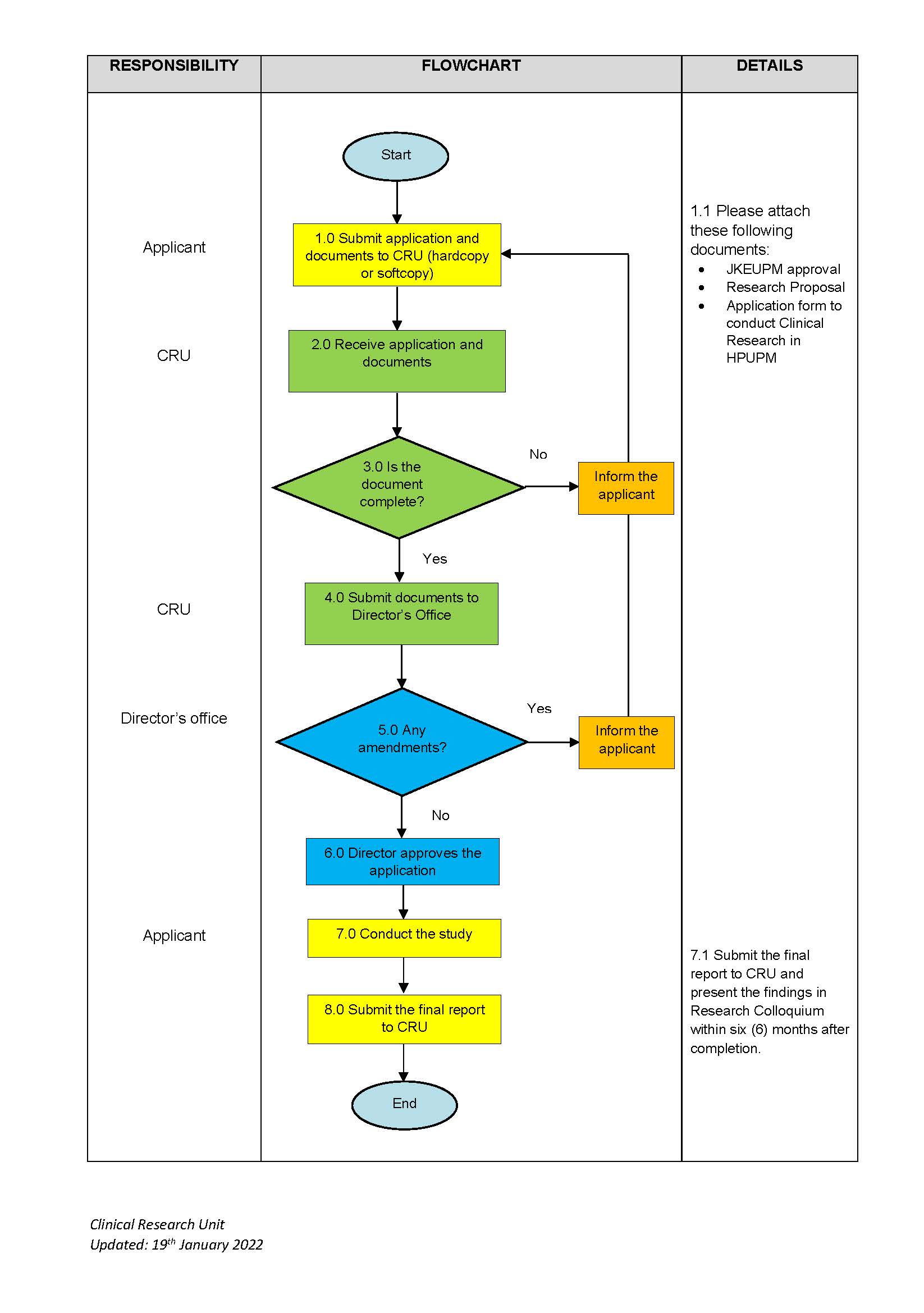
Researcher who want make application to conduct research at Hospital Sultan Abdul Aziz Shah (HSAAS) must prepared research document and submit the document (as a softcopy) to Clinical Research Unit, HSAAS for review and further action.
- Researchers need to download the following documents and attach supporting documents:
Document:
- Senarai Semak Permohonan Penyelidikan Klinikal

- Clinical Research Application Form

- JKEUPM Approval (Ethic Committee For Research Involving Human Subject)
- Research Proposal (FINAL)
- Every clinical research application at HSAAS must obtain written approval from the head of department for each facility used to conduct research (official letter/email)
- Request cooperation researchers to ensure:
- If the researcher's application is from outside the HSAAS, please obtain the Site Investigator (HSAAS internal researcher) as a co-investigator before submitting the research application document to the CRU.
For the purpose of JKEUPM Approval (Ethic Committee For Research Involving Human Subject). Please refer to the JKEUPM secretariat at the following link: JKEUPM
|
|
FLOWCHART OF APPLICATIONS FOR CONDUCTING RESEARCH WITHOUT AGREEMENT IN
HOSPITAL SULTAN ABDUL AZIZ SHAH (HSAAS)
TERMINOLOGY AND GLOSSARY:
CRU - Unit Penyelidikan Klinikal / Clinical Research Unit
JKEUPM - Jawatankuasa Etika Universiti Putra Malaysia
|
Updated:: 25/04/2024 [intanbasirah]
MEDIA SHARING