|
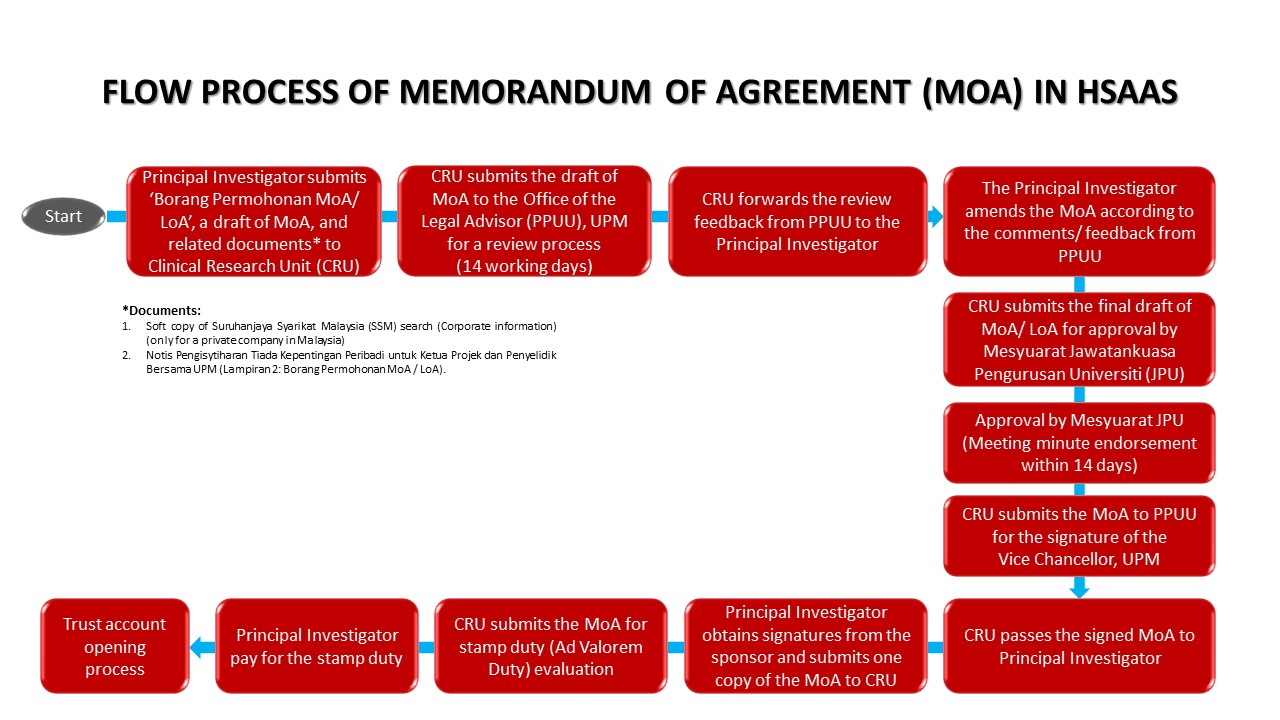
Researcher who want conduct Clinical Trial research at HPUPM must make application to Clinical Research Unit with prepared research document and submit the document (as a softcopy) to Clinical Research Unit office, for review and further action.

*Each application for clinical research at HSAAS must obtain approval from the head of department for each facility used*.
*If the application is from outside HSAAS, please get the Site Investigator first before sending the research application to CRU*.
Document:
- Senarai Semak Permohonan Penyelidikan Klinikal

- Clinical Research Application Form

- Application of JKEUPM (Ethics Committee for Research Involving Human Subjects) Approval Form

- MOA Application Form

- Application Form for Opening of Trust Account

- TOR of Clinical Trial Agreement (CTA) in HPUPM

|
|
|
|
|
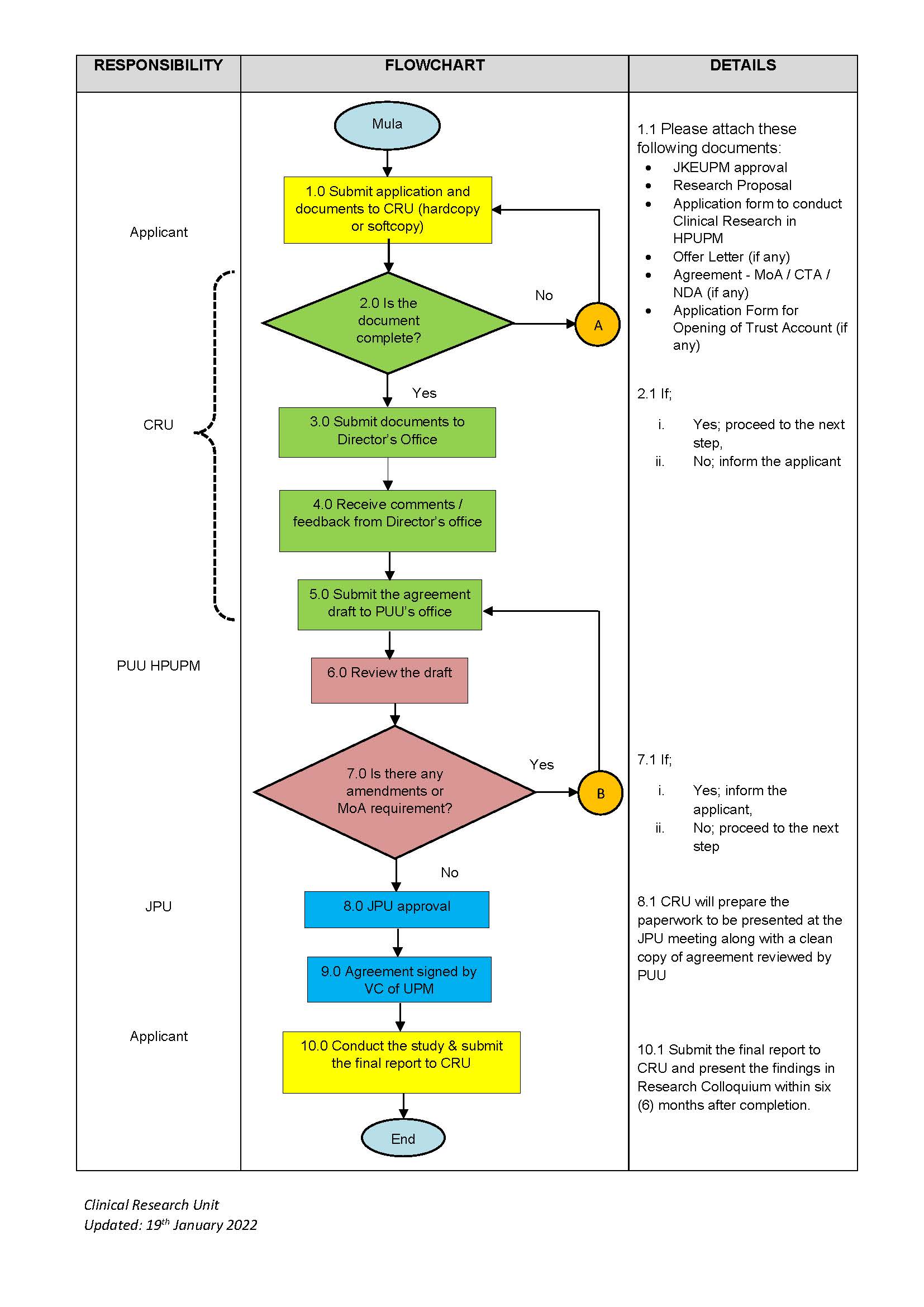
FLOWCHART OF APPLICATIONS FOR CONDUCTING RESEARCH WITH AGREEMENT IN
HOSPITAL SULTAN ABDUL AZIZ SHAH (HSAAS)
TERMINOLOGY AND GLOSSARY:
CRU – Unit Penyelidikan Klinikal / Clinical Research Unit
CTA – Clinical Trial Agreement
JKEUPM - Jawatankuasa Etika Universiti Putra Malaysia
JPU – Jawatankuasa Pengurusan Universiti
MoA – Memorandum Perjanjian / Memorandum of Agreement
PUU – Pegawai Undang-Undang
|
|
Updated:: 25/04/2024 [intanbasirah]
MEDIA SHARING